Abstract
Multiple myeloma (MM) is an incurable cancer of plasma cells (PC), with a median survival of 5-7 years. Osteolytic bone disease and skeletal complications occur in more than 80% of MM patients and significantly contribute to the morbidity and mortality of these patients. Glycosphingolipid (GSL), an essential constituent of the outer leaflet of the cellular membrane, is altered in MM and other hematological cancers. We previously reported that GM3, a subtype of GSL promotes osteoclastogenesis. On the other hand, the GSL synthase inhibitor N-butyl-deoxynojirimycin (NB-DNJ) reduces myeloma bone disease in the 5TGM1 mouse model of MM. Mechanistically, NB-DNJ prevents osteoclast (OC) development and activation by disrupting RANKL-induced localization of TRAF6 and c-SRC into lipid rafts and preventing nuclear accumulation of the transcriptional activator NFATc1.
Although NB-DNJ is an FDA-approved drug treating Gaucher's disease, it has many undesired off-target effects, such as inhibiting lysosomal and plasma membrane Beta-glucocerebrosidase and interfering with intestinal glucosidases which leads to gastrointestinal toxicities and severe weight loss. Therefore, more specific GSL inhibitors are required to minimize the side effects.
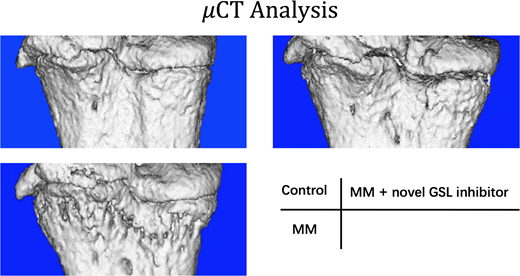
Here we report a novel GSL inhibitor called Genz112638 with comparable effects as NB-DNJ but reduced side effects. Genz112638 inhibits both OC formation (p < 0.01) and MM cell growth (p < 0.0001) in vitro in a dose-dependent manner. Moreover, compared to NB-DNJ, Genz112638 more significantly improved bone condition and potentially reduced MM burden, as evidenced by the amelioration of bone loss in the 5TGM1 model of myeloma, and a reduction in the proportion of MM within bone marrow and spleen without obvious adverse effects (n=6) (p < 0.01).
As excessive malignant PC in MM normally arise from germinal centre, we also checked the effects of Genz112638 on germinal centre reactions in wildtype mice. We found that Genz112638 suppresses the formation of germinal centre B cells in mouse spleen induced by sheep red blood cells (n=7). Thus, Genz112638 may affect the pathogenesis of MM disease at the initial stage.
Taken together, our data elucidate a novel specific GSL inhibitor as a promising candidate drug relieving two main features of MM: bone destruction and tumour burden with negligible side effects. In vitro, it decreases OC differentiation and proliferation, and meanwhile decreases MM viability and proliferation. In vivo, it may suppress B cell formation in germinal centre, ameliorate bone destruction, and potentially interfere with the vicious cycle between increased OC and susceptibility to MM. In short, we provide a preclinical platform for GSL inhibition as a new tool against MM and its related complications.
Horwood:Genzyme: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal